On the Influence of Polymeric Nature of Guanidine-containing Fntiseptics on their Biocidal Properties and Toxicity
The basic factors that influence the choice of antiseptics are its efficiency against pathogenic microorganisms, its toxicity, activity duration, and convenience of application.
The comparative analysis of high-molecular and low-molecular compounds allows concluding that polymeric biocides have the best characteristics over the total range of parameters.
A rather wide range of natural and synthetic compounds has antimicrobial properties. However, most of those substances are not safe for humans and animals. This is why only a few chemicals can be recommended for practical application as antiseptics.
Polymers containing nitrogen atoms in backbone or side chains (cationic polyelectrolytes) and their low-molecular analogues are among such chemicals. These compounds can impact the cell membrane of the bacteria so as to kill the latter.
Structure of the bacterial cell membrane
Bacterial cell membrane consists of a cell wall and a cytoplasmic membrane. It provides osmotic barrier and selective transport of substances into the cell. Small molecules, ferments, and exotoxins can penetrate the membrane.
Phosphate groups of lipid molecules, as well as sialic and teichoic acids are grouped on the cell wall surface; this results in the negative net charge of the bacterial cell.
Cytoplasmic membrane of the cell ensures the constancy of cell’s composition; its integrity is a necessary condition of the cell’s existence. The membrane consists of phospholipids and proteins.
The electronegative hydrophilic parts of polar phospholipid molecules are aligned on the outside of the membrane, whereas the hydrophobic ones (fatty acid residues) form rows of parallel hydrocarbon chains on the inside of the membrane.
The electrostatic interaction of polar groups, as well as hydrophobic interactions of proteins and lipids makes this structure stable.
Mechanism of interaction of cationic polyelectrolytes and the bacterial cell
The interaction of cationic polyelectrolytes and their low-molecular analogues (that have a positive net charge) with negatively charged bacterial cell passes the following stages.
During the first stage the cations are adsorbed by the cell surface thus blocking cell respiration, nutrition, and transport of metabolites through the cell wall. Then the polyelectrolytes disrupt the cell wall and penetrate the cell, and then they start interacting with the phospholipids and proteins of the cytoplasmic membrane. These processes cause destabilization of the electrostatic and hydrophobic interactions within the membrane, disorganize its structure, and finally lead to its rupture, blocking of respiratory system and death of the microbe cell.
Influence of the macromolecular nature of cationic polyelectrolytes
The macromolecular nature of cationic polyelectrolytes contributes to the change in the reaction ability in its interaction with a microbe cell. This change is due to the following factors: cooperative interaction of the functional group neighbouring along the chain, intramolecular interactions of remote fragments of the chain, conformational transformations of the polymeric chain, changes in complementarity of the biocide molecules and the protein molecules in the bacterial cell.
Afinogenov and Panarin studied the interaction of microbe cells with polyelectrolytes, which have flexible backbones. They have shown that the antimicrobial activity of a polymeric reagent depends primarily on the value of the charge carried by the macromolecule.
Co operative binding of positively charged macromolecules with the negatively charged bacterial cell (many-point adsorption) increases the antibacterial activity of the polycation compared with the corresponding monomers or low-molecular compounds (however, there are exceptions to this rule).
The chemical structure of the hydrocarbon radicals of the polymeric reagent also influences the chemical activity of the polycation in its interaction with the microbe cell.
These radicals participate in the hydrophobic interactions of the non-polar part of the cell membrane thus changing the surface activity and lipophility of the compound.
Quaternary ammonium compounds and polyguanidines belong to the best-known polymeric cationic polyelectrolytes.
Guanidine antiseptics
Guanidine compounds are widespread in nature and find application as physiologically active agents: drug substances, antiseptics, fungicides, pesticides, etc. Amino acid arginine, folic acid, as well as many nucleic acids and proteins belong to this class of compounds.
Guanidine group is the active component of many antibiotics (streptomycin, etc.) and other pharmaceutical substances (sulginum (sulfaguanidine), ismelin (guanethidine), Faringosept, aspirin).
The guanidinium cation interacting with the microbe cell causes high biocidal activity of guanidine compounds. In contrast to the quaternary ammonium compounds, where the positive charge is localised near a single nitrogen atom (1), in guanidinium cation the charge is distributed between three such atoms (2).
Such structure of the reaction centre ensures the necessary equilibrium between the efficiency of biocide action and the toxicity towards warm-blooded animals and humans.
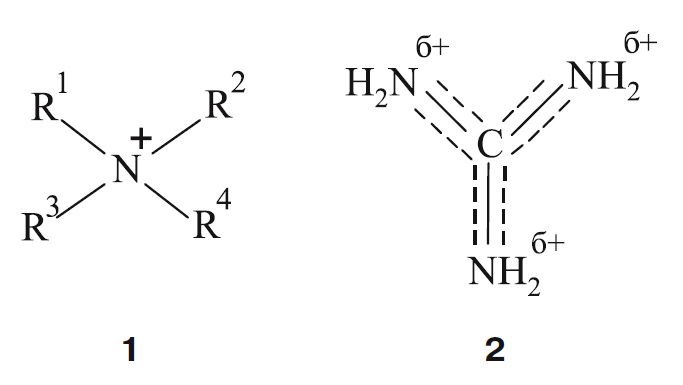
In order to clarify the influence of the polymeric nature of guanidine antiseptics on their efficiency against microorganisms and toxicity towards humans, we compare the properties of a typical polyguanidine, namely, PHMG, i.e. polyhexamethylene guanidine (3), and its low-molecular analogue chlorhexidine.
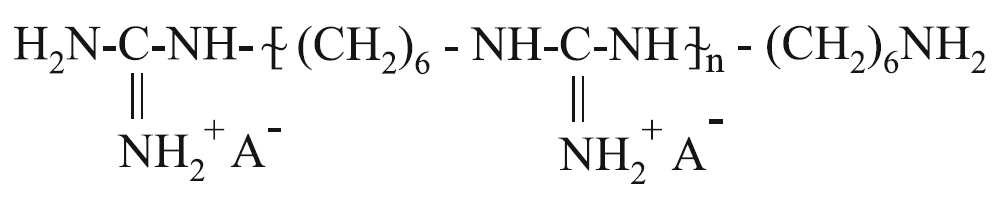
where n = 5-90; А- is an anion of an organic or an inorganic acid.
High-molecular salts of PHMG are solid substances soluble in water. They have the properties of a cationic polyelectrolyte and a strong organic base. They are effective against many pathogenic microorganisms causing purulent, respiratory, intestinal, venereal, and other diseases of humans and animals, and are low toxic for humans.
Chlorhexidine is odourless white crystalline powder poorly solved in water and alcohol. Therefore its derivative chlorhexidine digluconate (4) well soluble in water is used in pharmaceutical formulations:
Comparing the efficiency of biocidal activity.
Chlorhexidine digluconate has a strong and immediate bactericidal action; therefore it is often applied as an effective disinfectant in medical practice. It is used for prevention of nosocomial infections, prophylactics of venereal diseases, sterilisation of surgical instruments, disinfection of operative site, surgeon’s hands, treatment of operative wounds, urinary bladder, burns, etc.
Biocidal activity of chlorhexidine is limited: some of the nosocomial microbe cultures are immune to its action. Chlorhexidine has low efficiency against tuberculosis mycobacteria, pseudomonas cultures, and aerobic bacteria. It does not impact viruses and spores. Efficiency of this disinfectant is still decreased by the presence of proteins.
The available experimental data (Figure 1) show that PHMG salts are more effective against many bacteria and fungi than chlorhexidine digluconate.
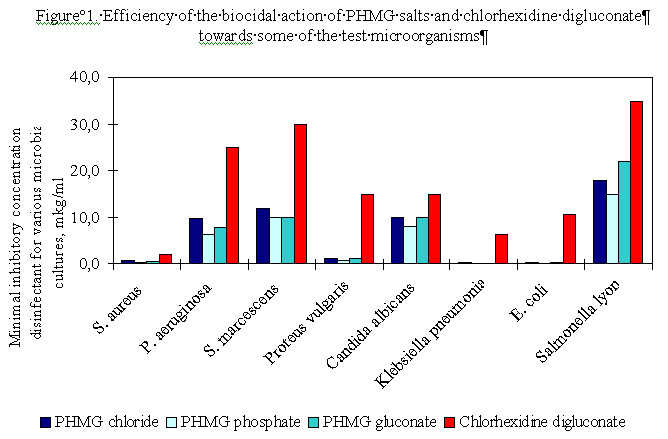
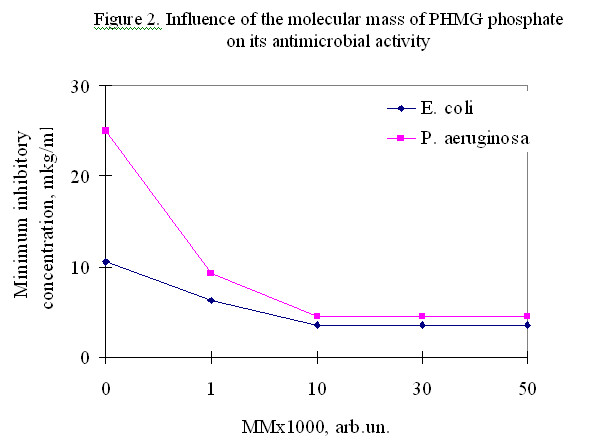
Figure 1 shows that the minimum inhibitory concentrations of the best-studied PHMG salts are similar, i.e. their biocidal activity is approximately the same. Figure 2 shows that the increase of molecular mass (MM) of PHMG leads to a decrease of the minimum inhibitory concentration reaching a maximum at MM = 10000.
According to the available experimental data, further increase of the PHMG molecule size does not cause any increase of the antimicrobial activity; Japanese researchers found a certain activity growth up to the value of MM ~ 80 000.
Macromolecular nature of the PHMG ensures a long-term antimicrobial activity of the disinfectant. In contrast to the low-molecular compounds, which ensure antimicrobial activity only for several hours (several days, at best), polymer forms a biocidal film on the treated surface, which ensures a long-term (several months) protection of the treated surface from microorganism propagation.
The presence of a thin polymeric film formed on the surface treated with 1% solution of Biopag (PHMG chloride) was experimentally proven with X ray photoelectronic spectroscopy (XPS).
It was found that the polymeric film remains on the treated surface for several months and even after 6 months retains its biocidal activity.
It was also shown that, compared to chlorhexidine, PHMG derivatives have a wider range of biocidal activity. PHMG disinfectants are equally efficient against aerobic and anaerobic microflora, including Pseudomonas aeruginosa and mycobacteria tuberculosis; they suppress pathogens of many especially dangerous diseases (legionellosis, glanders, and plague); they have virucidal activity against pathogens of poliomyelitis, AIDS, hepatites, herpes, and flue.
Thanks to their polymeric nature, PHMG has the properties of a cationic flocculant and can therefore be used in water treatment technology both as biocide and flocculant. This allows sparing special flocculants and using two reagents (coagulant and biocidal flocculant) instead of three (coagulant, flocculant, and biocide). This also allows replacing chlorine and chlorine containing disinfectants in water treatment technology, because they form highly toxic organochlorine compounds in the treated water.
The combination of properties of polyguanidines allows extending the application range of these disinfectants. Apart from the obvious use as antiseptics in medical practice, they can also be used as biocidal additives to various materials (cement mortar, rubber, fabrics, paper pulp, paints, etc.), and as auxiliary materials in various technological processes.
Comparison of toxic properties
The experimental data accumulated over recent years prove that PHMG derivatives are less toxic than their low-molecular analogues. In particular, for warm-blooded animals the average lethal dose (LD50) of most of PHMG derivatives exceeds that of chlorhexidine digluconate for all exposure types.
One of the reasons for toxicity reduction in case of polyguanidines consists in different skin penetrability for polymers and their low-molecular analogues. Experiments have shown that polyguanidines can be adsorbed through undamaged skin. However, due to the small value of oil / water distribution factor, the rate of transdermal resorbtion through undamaged skin for polymers is much lower than for their low-molecular analogues (see Table 1).
After drying the polymer forms a film on the skin surface; this film prevents further resorbtion of the antiseptic, therefore transcutaneous penetration of the polymer rapidly diminishes. Low mobility of large molecules and impenetrability of cell membranes of warm-blooded animals to these antiseptics can also contribute to the decrease of the polymer’s toxicity.
The toxicity of polyguanidines essentially depends on the chemical nature of the anion A-. In order to exclude the influence of anion and to clarify the influence of polymeric structure of guanidine-containing antiseptic on its toxicity, the nearest high- and low-molecular analogues, namely, PHMG gluconate and chlorhexidine digluconate will be compared.
Table 1 shows some of the toxicity parameters of these antiseptics for transcutaneous exposure of warm-blooded animals. We can see that all the threshold concentrations of the polymeric biocide, as well as its maximum allowable concentration are considerably higher than similar characteristics of its low-molecular weight analogue are. Thus the toxicity of polymeric antiseptics is lower than that of similar compounds with low molecular weight.
According to the toxicity parameters, both these substances (as well as other PHMG salts) belong to the class of low-hazard chemicals. However, the certain safety factor (which shows the ratio of toxicity of the agent towards pathogenic microflora and towards humans) is much higher for PHMG gluconate than for chlorhexidine digluconate (Table 1). The average value of the CSF for various PHMG salts is 3667.
| Parameter | Chlorhexidine digluconate | PHMG gluconate |
|---|---|---|
| Transdermal resorbtion rate ГК/cm/hour | 10.3 | 1.5 |
| Acute action threshold, mg/kg | 1000 | 2500 |
| Threshold of skin irritating action, mg/kg | 20 | 500 |
| Neurotoxic action threshold, mg/kg | 400 | Not found |
| Threshold of chronic general toxic action , mg/kg | 58 | 150 |
| Threshold of chronic general toxic action , mg/kg | 0.02 | 0.11 |
| Certain safety factor (CSF) | 750 | 6250 |
Practical application of chlorhexidine digluconate in some cases causes skin xerosis, itch, and dermatitis; PHMG does not cause allergic reactions of skin.
The toxicity of polyguanidines (average lethal dose LD50) decreases with the decrease of electronegativity of anion A-. Barkova and Bogachuk have developed a theory based on the available experimental data and the results of quantum mechanical calculations. According to this theory, toxic properties of guanidine compounds depend on the degree of positive charge delocalization in the molecule.
The influence of the anion is explained by its donor-acceptor properties. Anionic acceptors (e.g., Cl-, OH-) attract the electrons thus increasing the positive charge of the guanidinium anion. This redistribution of electronic density in the guanidine group causes stress in the whole macromolecule.
The molecule then assumes a stretched conformation so that all the guanidine groups are easily accessible and can realise their reaction ability in interaction with a cell.
Anions of gluconic, and other organic acids, on the opposite, facilitate transfer of the electronic density towards the guanidine group, thus decreasing its positive charge. This redistribution of electronic density initiated by the anion propagates along the polymeric chain and enhances the intramolecular interactions of remote functional groups of the chain.
This results in the macromolecule assuming a spiral conformation, which is stabilised by hydrogen bonds and the interaction of hexamethylene fragments (anion determines the pitch of the molecular spiral). Part of the guanidine groups is therefore blocked and loses the reactivity. Besides, complementarity of the polyguanidine molecule and the receptors of the cell membrane structures can be disrupted.
Thus a PHMG macromolecule (3) is a well-balanced system where the guanidine group carries a positive charge and ensures bactericidal properties. Anion A- impacts the degree of electron charge delocalization and thus controls toxicity.
Hexamethylene chain facilitates electronic density transfer in the macromolecule and also regulates hydrophilic-hydrophobic equilibrium of the molecule.
Thus, enhancement of the biocidal efficiency of the polymer in comparison to the low-molecular analysis is due to the co-operative interaction of multiple chemically bound guanidine groups with the microbial cell. Toxicity reduction is due to the possibility of electronic density delocalization along the polymeric chain and the conformational changes in the latter.
Chemical modification
Polymeric nature of the antiseptic opens wide possibilities of its chemical modification, synthesis of guanidine polymers with various linear and spatial structures, and control of its physical properties, biocidal action, and toxicity.
Chemical nature of anions in the repeating units of the macromolecule can be changed, polymethylene chain length can be controlled, heteroatoms (e.g., oxygen bridges) can be included into the polymeric chain.
Because of high reactivity of guanidine groups, PHMG can react with various bifunctional and polyfunctional compounds including polymers. Such reactions can lead to changes in solubility, ability of film formation, as well as in polymeric film strength, and other physico-chemical characteristics of the polymeric antiseptic.
Biocides with low molecular mass lose their antimicrobial properties after any chemical reaction impacting the biologically active centres, whereas polymers preserve their antiseptic properties in most such cases.
The reason for this is that such reactions in polymeric chains never reach 100% conversion due to the conformational and steric factors. Therefore modified PHMG always contains free (unreacted) guanidine groups. Thus, by controlling the conversion of functional groups of the polymer during the chemical reaction, the optimal combination of biocidal, toxic, and physico-chemical properties of the antiseptic can be achieved.
The Institute of Ecotechnologies used chemical modification of PHMG for creation of various high-molecular biocides for different applications. Below several most remarkable of them are listed and discussed. Ecosept has very low toxicity and good antiseptic properties, and Gembicide is antimicrobial chemical with enhanced antiseptic properties and high hydrophobicity.
Polymeric antiseptics with enhanced antitubercular activity, which are able to penetrate the hydrophobic wax-like shell of tuberculosis mycobacteria, were synthesised based on various derivatives of PHMG.
Organosoluble film-forming interpolymer Septopag has very remarkable properties. It forms stable waterproof biocide coating on the surface of the treated objects. These coatings have very high strength, which is characteristic for the film-forming chlorsulfonated polyethylene.
Physico-chemical properties and stability of PHMG
Here we would like to note that chlorhexidine digluconate is produced outside Russia via complex multi-stage technology involving highly toxic halogen-cyan compounds. In contrast to this chemical, the variety of PHMG derivatives is produced at the experimental plant of the Institute of Ecotechnologies using only low-toxic and easy-to-handle raw materials.
PHMG salts have a convenient commercial product form: they are mostly solid substances without taste or odour, stable against oxidative and thermal destruction, and ageing (they preserve their biocidal activity for at least 15 years). These polymers are well soluble in water; 20% water solutions preserve their biocidal activity for at least 5 years.
In addition to the biocidal properties, PHMG has the properties of surfactants and cationic polyelectrolytes. Therefore apart from disinfection, they also find application as biocidal additives to various materials, and auxiliary substances in a wide range of technological processes, including galvanic technology, optical glass polishing, paper production, etc.
Irina Vointseva,
Dr.Sci. (chemistry)
Nikolay Polikarpov,
Ph.D. (biology)